We ensure strict adherence to regulatory standards and safe handling of controlled substances. With meticulous attention, we manage storage, transportation, and documentation, preventing diversion or misuse. Our commitment to stringent control and security protocols guarantees lawful distribution to global healthcare providers and research institutions.
Reference Drug- The Future of Generic Drugs
The global generics drug market is anticipated to grow to $574.63 billion by 2030 owing to the increasing application of robotic process automation, branded medicine patent expiries, and the rising prevalence of chronic diseases. New generic versions are compared to approved drug products known as Reference Listed Drugs (RLDs) to demonstrate their bioequivalence. A pharmaceutical business seeking to offer a generic equivalent must cite the Reference Listed Drug in its regulatory applications. Invimeds sources and provides RLDs globally. We have an excellent network of validated suppliers across the EU, USA, Brazil, Korea, Latam, and Japan and can procure pharmaceutical products directly and securely for most major pharmaceutical companies.
Solution & Offering- RLD Services
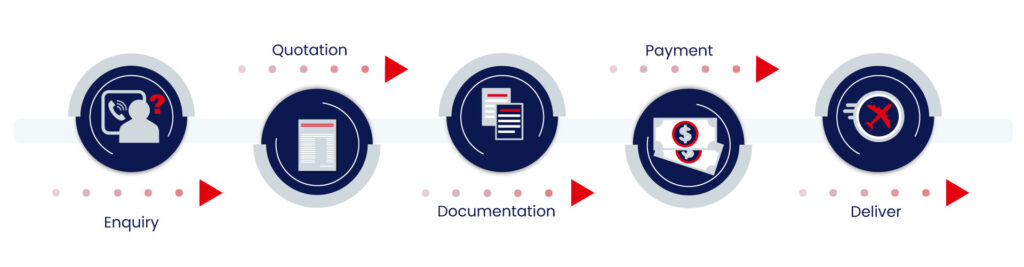
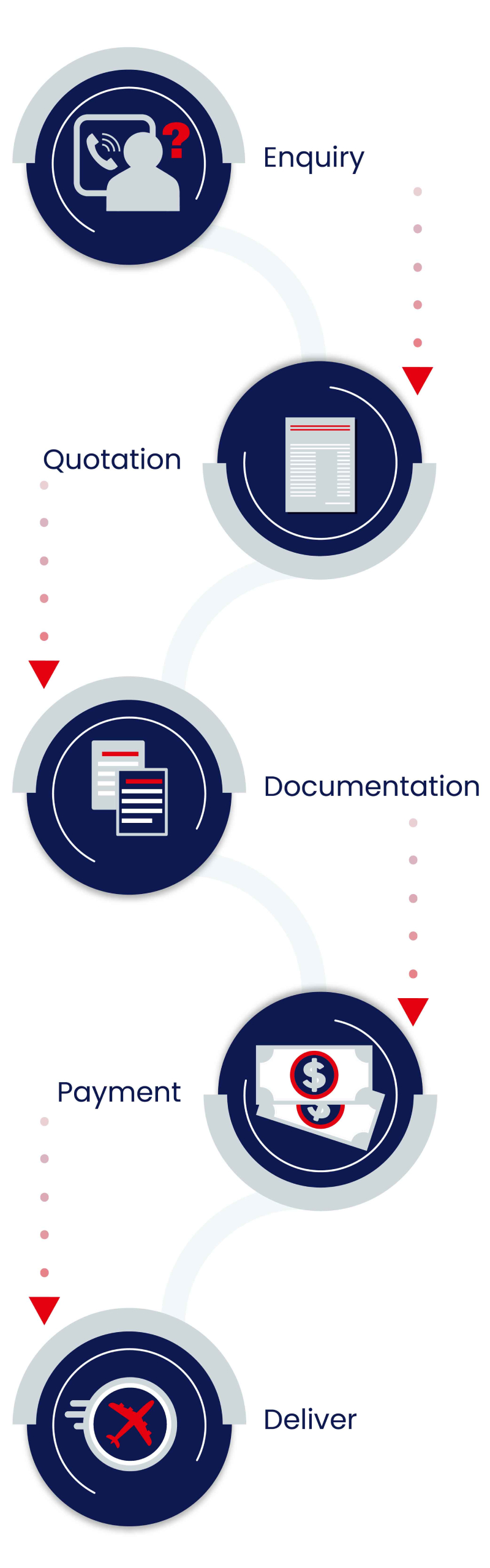
Our Process
Why you should trust InviMeds for Reference Drug sourcing?
Catering to more than 75+ Pharma Companies
Currently catering to more than 25+ major players and helping 75+ companies in the healthcare industry with controlled drugs or biologics or Innovators.
Registered with 40+ Pharma Companies
Registered with 40+ Pharma Companies as an approved vendor to their organization
EU-GDP Certified Warehouse
We operate an EU-certified warehouse, which meets EU GDP guidelines, and follows a set of Standard Operating Practices(SOPs) written according to mandates provided by the EU.
Well-established Network
We have a well-established network of suppliers in the USA, EU, Japan, Netherlands, Australia, South Korea, etc that have been carefully validated and authenticated.
Regular Product Updates
To keep you informed and help you plan your study appropriately, our team will regularly provide updates and advise of product shortages or manufacturer delays.
Logistics for Cold Storage & Shipment
Our partnership with leading logistic companies for cold storage and shipment, as well as routine deliveries, ensures that products are delivered at the client warehouse or the landing port without temperature excursion
FAQs
Does Invimeds supply controlled products from the USA and EU?
Yes, we supply controlled products from the USA, EU(Belgium, Bulgaria, Germany, France, Latvia, Poland, Netherlands, etc), Australia, South Korea, and Japan. We are a pharmaceutical sourcing company with the authorized license to acquire a comprehensive range of controlled products from these regions. We adhere strictly to regulatory guidance and standards to ensure the legality, safety, and authenticity of the products we distribute. Our commitment to following established regulations and implementing stringent quality control measures makes us a trustworthy distributor of controlled substances.
What are Reference Listed Drugs (RLDs) and how significant are they in the pharmaceutical industry?
RLDs (Reference Listed Drugs) are brand-name medications that have been authorized by regulatory agencies. They serve as the standard for generic drug makers, ensuring the safety, effectiveness, and quality of generic medications. RLDs are critical to the pharmaceutical industry’s ability to maintain uniform standards.
How does Invimeds Health obtain RLDs for pharmaceutical companies and clinical trials?
Invimeds Health has formed alliances with respected manufacturers and suppliers all around the world. We leverage our wide network to quickly procure RLDs for pharmaceutical firms and clinical studies. Our stringent quality controls assure the medications’ validity and dependability.
Can Invimeds Health help through the regulatory requirements for RLD supplies?
Yes, Invimeds Health provides regulatory support for RLD suppliers. We have a team of professionals who are familiar with legal pharmaceutical standards, and we can help pharmaceutical companies meet the standards for procuring and employing RLDs in their products or clinical studies.
Are you Supplying globally?
Yes, Our company is dedicated to the global supply of Reference or Innovator drug.
What all documents you can provide to client?
We can provide documents including COA(Certificate of Analysis), COC(Certificate of Conformity), COO(Certificate of Origin), Packing list, and Country invoices.
Speak with Experts
At Invimeds Health, we believe in offering innovative strategies for an unparalleled experience. Communicate with our experts and learn more about how we can help you with our expertise in Reference Listed Drugs procurement. We follow the Standard Operating Procedures to ensure hassle-free trials. Our team of qualified experts possess years of knowledge in the RLD industry catering to the comprehensive needs of the customers.
Get in touch
Have an inquiry related to RLD sourcing or some feedback for us? Fill out the form below to contact our team